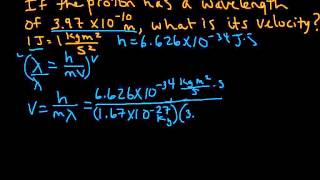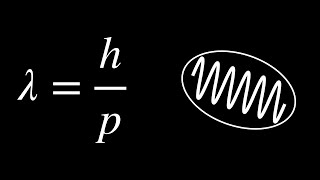9. Quantum Mechanics
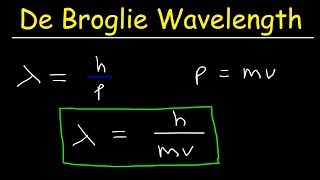
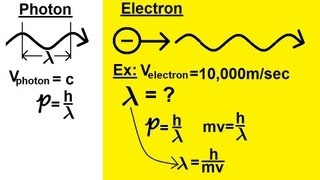
De Broglie Wavelength
Problem 136
Textbook Question
Textbook Question(b) Light with a wavelength of 2.50 * 10-7 m falls on a piece of chromium in an evacuated glass tube. What is the minimum de Broglie wavelength of the emitted elec-trons? (Note that the energy of the incident light must be conserved; that is, the photon's energy must equal the sum of the energy needed to eject the electron plus the kinetic energy of the electron.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
457
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos