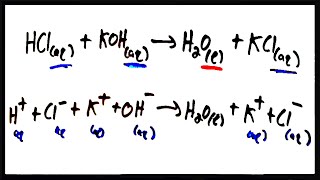6. Chemical Quantities & Aqueous Reactions
Complete Ionic Equations
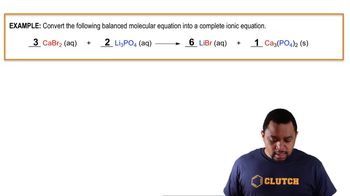
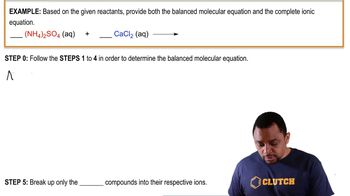
Problem 121
Textbook Question
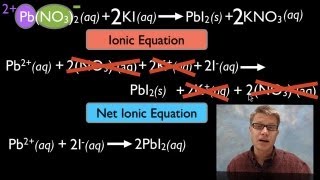
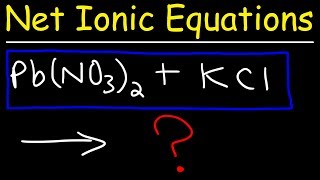
Textbook QuestionA solution contains one or more of the following ions: Ag+ , Ca2 + , and Cu2 + . When you add sodium chloride to the solution, no precipitate forms. When you add sodium sulfate to the solution, a white precipitate forms. You filter off the precipitate and add sodium carbonate to the remaining solution, producing another precipitate. Write net ionic equations for the formation of each of the precipitates observed.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
3036
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos