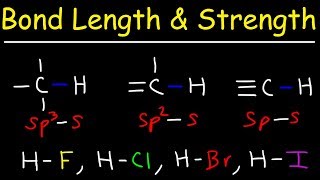11. Bonding & Molecular Structure
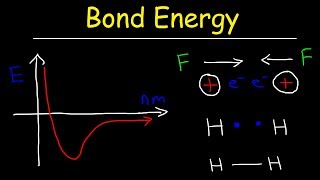
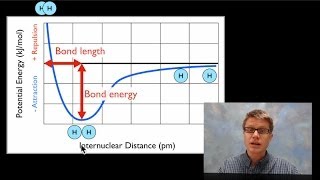
Bond Energy
Problem 105c
Textbook Question
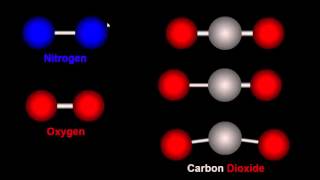
Textbook QuestionIf hydrogen were used as a fuel, it could be burned according to this reaction: H2( g) + 1 2 O2( g)¡H2O( g) Which fuel yields more energy per mole?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
12mPlay a video:
344
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos