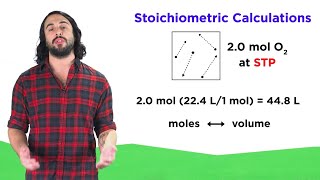
7. Gases
Gas Stoichiometry
Problem 105
Textbook Question
Textbook QuestionAmmonium carbonate decomposes upon heating according to the balanced equation: (NH4)2CO3(s)¡ 2 NH3( g) + CO2( g) + H2O( g) Calculate the total volume of gas produced at 22 °C and 1.02 atm by the complete decomposition of 11.83 g of ammonium carbonate.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
7417
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos