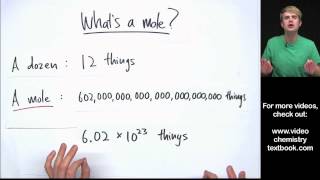2. Atoms & Elements
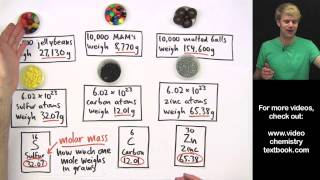
Mole Concept
Problem 84d
Textbook Question
Textbook QuestionWhat is the mass, in grams, of each elemental sample? a. 2.3 * 10 - 3 mol Sb
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
48sPlay a video:
242
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 15 videos