9. Quantum Mechanics
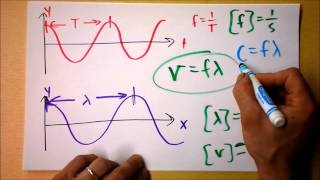
Speed of Light
Problem 109b
Textbook Question
Textbook QuestionMoseley established the concept of atomic number by studying X rays emitted by the elements. The X rays emitted by some of the elements have the following wavelengths: Element Wavelength (pm) Ne 1461 Ca 335.8 Zn 143.5 Zr 78.6 Sn 49.1 (e) A particular element emits X rays with a wavelength of 98.0 pm. What element do you think it is?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
12mPlay a video:
353
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos