6. Chemical Quantities & Aqueous Reactions
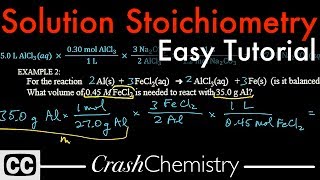
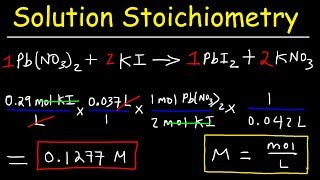
Solution Stoichiometry
Problem 147
Textbook Question
Textbook QuestionA mixture of FeCl2 and NaCl is dissolved in water, and addi-tion of aqueous silver nitrate then yields 7.0149 g of a pre-cipitate. When an identical amount of the mixture is titrated with MnO4 -, 14.28 mL of 0.198 M KMnO4 is needed for complete reaction. What are the mass percents of the two compounds in the mixture? (Na+ and Cl-do not react with MnO4 -. The equation for the reaction of Fe2+ with MnO4 was given in Problem 4.146.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
845
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos