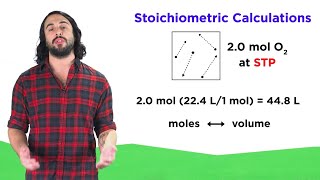
7. Gases
Gas Stoichiometry
Problem 91
Textbook Question
Textbook QuestionGaseous compound Q contains only xenon and oxygen. When 0.100 g of Q is placed in a 50.0-mL steel vessel at 0 °C the pressure is 0.229 atm. (b) When the vessel and its contents are warmed to 100 °C, Q decomposes into its constituent elements. What is the total pressure, and what are the partial pressures of xenon and oxygen in the container?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
11mPlay a video:
392
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos