15. Chemical Kinetics
Integrated Rate Law
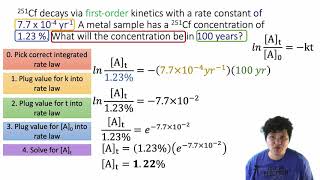
Problem 50
Textbook Question
Textbook QuestionSucrose 1C12H22O112, commonly known as table sugar, reacts in dilute acid solutions to form two simpler sugars, glucose and fructose, both of which have the formula C6H12O6. At 23 C and in 0.5 M HCl, the following data were obtained for the disappearance of sucrose: Time (min) 3C12H22o11 4 1M2 0 0.316 39 0.274 80 0.238 140 0.190 210 0.146 (a) Is the reaction first order or second order with respect to 3C12H22O114?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
2319
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos