7. Gases
Standard Temperature and Pressure
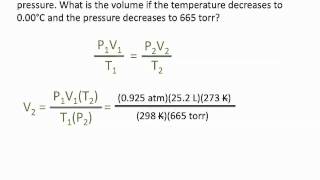
Problem 13a
Textbook Question
Textbook QuestionAt standard temperature and pressure, the molar volumes of Cl2 and NH3 gases are 22.06 and 22.40 L, respectively. (b) On cooling to 160 K, both substances form crystalline solids. Do you expect the molar volumes to decrease or increase on cooling the gases to 160 K?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
457
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos