1. Intro to General Chemistry
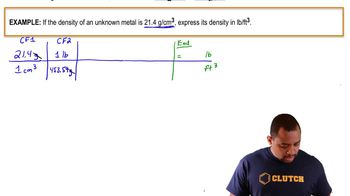
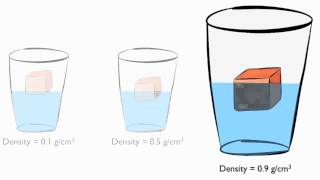
Density

Get help from an AI Tutor
Ask a question to get started.
Problem 81
Textbook Question
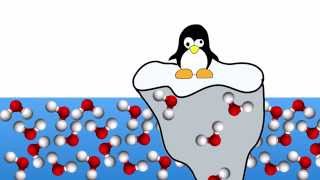
Textbook QuestionWater has a density of 0.997 g>cm3 at 25 C; ice has a density of 0.917 g>cm3 at -10 C. (a) If a soft-drink bottle whose volume is 1.50 L is completely filled with water and then frozen to -10 C, what volume does the ice occupy? (b) Can the ice be contained within the bottle?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
3844
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos