8. Thermochemistry
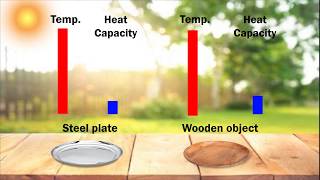
Heat Capacity
Problem 79a
Textbook Question
Textbook QuestionAssume that the kinetic energy of a 1400 kg car moving at 115 km/h (Problem 1.78) is converted entirely into heat. How many calories of heat are released, and what amount of water in liters could be heated from 20.0 °C to 50.0 °C by the car's energy? (One calorie raises the temperature of 1 mL of water by 1 °C)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
667
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos