2. Atoms & Elements
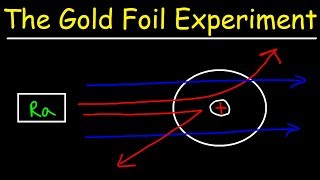
Rutherford Gold Foil Experiment

Get help from an AI Tutor
Ask a question to get started.
Problem 17
Textbook Question
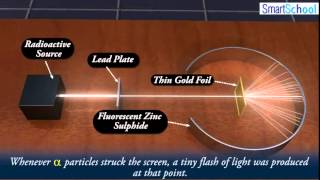
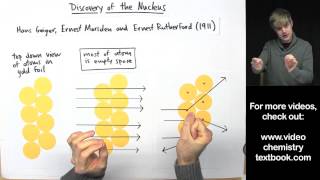
Textbook QuestionWhat fraction of the a particles in Rutherford's gold foil experiment are scattered at large angles? Assume the gold foil is two layers thick, as shown in Figure 2.9, and that the approximate diameters of a gold atom and its nucleus are 270 pm and 1.0 * 10–2 pm, respectively. Hint: Calculate the cross sectional area occupied by the nucleus as a fraction of that occupied by the atom. Assume that the gold nuclei in each layer are offset from each other.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
1224
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos