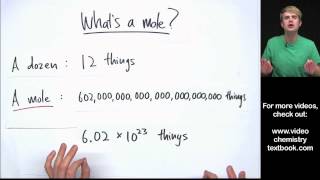2. Atoms & Elements
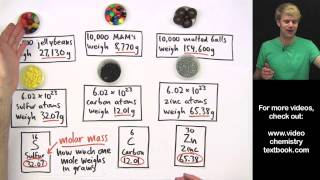
Mole Concept
Problem 117
Textbook Question
Textbook QuestionA copper wire having a mass of 2.196 g was allowed to react with an excess of sulfur. The excess sulfur was then burned, yielding SO2 gas. The mass of the copper sulfide produced was 2.748 g. (c) Calculate the number of copper ions per cubic centimeter if the density of the copper sulfide is 5.6 g/cm3.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
236
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 15 videos