8. Thermochemistry
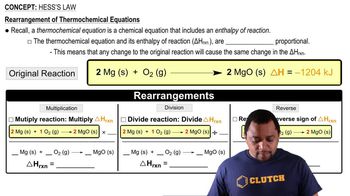
Hess's Law

Get help from an AI Tutor
Ask a question to get started.
Problem 155
Textbook Question
Textbook QuestionReaction of gaseous fluorine with compound X yields a sin- gle product Y, whose mass percent composition is 61.7% F and 38.3% Cl. (c) Calculate ΔH° for the synthesis of Y using the following information: 2 CIF1g2 + O21g2 S Cl2O1g2 + OF21g2 ΔH° = + 205.4 kJ 2 CIF31l2 + 2 O21g2 S Cl2O1g2 + 3 OF21g2 ΔH° = + 532.8 kJ OF21g2 ΔH°f = + 24.5 kJ>mol
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
286
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos