8. Thermochemistry
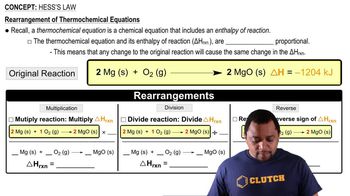
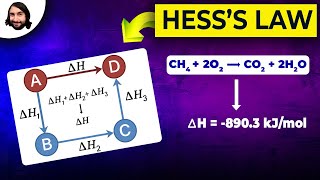
Hess's Law

Get help from an AI Tutor
Ask a question to get started.
Problem 110
Textbook Question
Textbook QuestionWe can use Hess's law to calculate enthalpy changes that cannot be measured. One such reaction is the conversion of methane to ethane: 2 CH41g2 ¡C2H61g2 + H21g2 Calculate the H° for this reaction using the following thermochemical data: CH41g2 + 2 O21g2¡CO21g2 + 2 H2O1l2 H° = -890.3 kJ 2 H21g2 + O21g2¡2 H2O1l2 H ° = -571.6 kJ 2 C2H61g2 + 7 O21g2¡4 CO21g2 + 6 H2O1l2 H° = -3120.8 kJ
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
847
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos