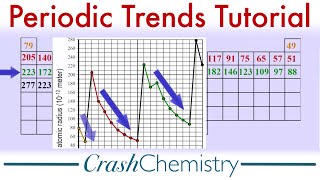
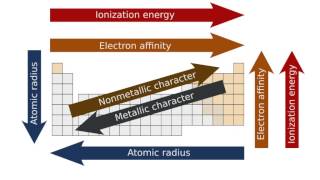
10. Periodic Properties of the Elements
Periodic Trend: Cumulative
Problem 54
Textbook Question
Textbook QuestionConsider the following equation: Al3+1g2 + e-¡Al2+1g2 Which of the following statements are true? (i) The energy change for this process is the second electron affinity of Al atom since Al2+1g2 is formed. (ii) The energy change for this process is the negative of the third ionization energy of the Al atom. (iii) The energy change for this process is the electron affinity of the Al2+ ion.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
685
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos