6. Chemical Quantities & Aqueous Reactions
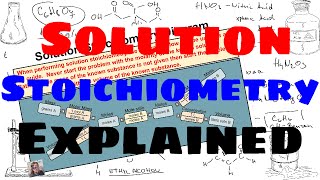
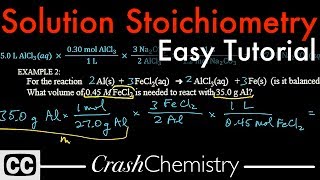
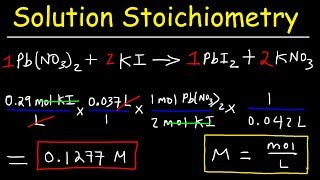
Solution Stoichiometry
Problem 70
Textbook Question
Textbook QuestionA 55.0-mL sample of a 0.102 M potassium sulfate solution is mixed with 35.0 mL of a 0.114 M lead(II) acetate solution and this precipitation reaction occurs: K2SO4(aq) + Pb(C2H3O2)2(aq)¡2 KC2H3O2(aq) + PbSO4(s) The solid PbSO4 is collected, dried, and found to have a mass of 1.01 g. Determine the limiting reactant, theoretical yield, percent yield.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
4845
views
4
rank
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos