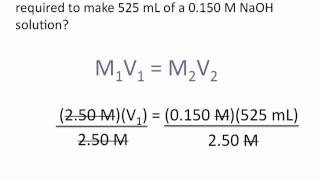6. Chemical Quantities & Aqueous Reactions
Dilutions

Get help from an AI Tutor
Ask a question to get started.
Problem 60b
Textbook Question
Textbook QuestionYou make 1.000 L of an aqueous solution that contains 35.0 g of sucrose (C12H22O11). (b) How many liters of water would you have to add to this solution to reduce the molarity you calculated in part (a) by a factor of two?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
745
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos