15. Chemical Kinetics
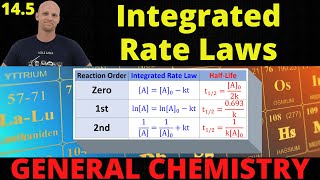
Integrated Rate Law

Get help from an AI Tutor
Ask a question to get started.
Problem 44
Textbook Question
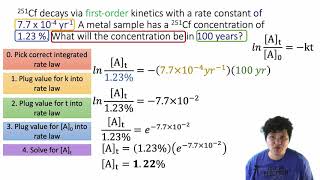
Textbook QuestionThe first-order rate constant for the decomposition of N2O5, 2 N2O51g2¡4 NO21g2 + O21g2, a t 70 C i s 6.82 * 10-3 s-1. Suppose we start with 0.0250 mol of N2O51g2 in a volume of 2.0 L. (a) How many moles of N2O5 will remain after 5.0 min?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
2444
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos