3. Chemical Reactions
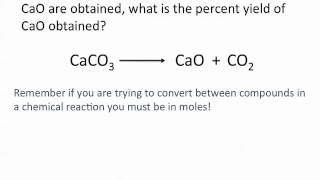
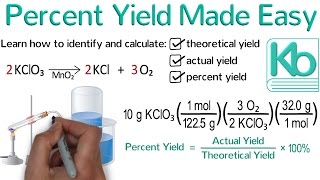
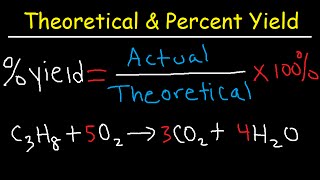
Percent Yield
Problem 108
Textbook Question
Textbook QuestionThe combustion of liquid ethanol (C2H5OH) produces carbon dioxide and water. After 4.62 mL of ethanol (density = 0.789 g>mL) is allowed to burn in the presence of 15.55 g of oxygen gas, 3.72 mL of water (density = 1.00 g>mL) is collected. Determine the percent yield for the reaction. (Hint: Write a balanced equation for the combustion of ethanol.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
3440
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos