15. Chemical Kinetics
Integrated Rate Law
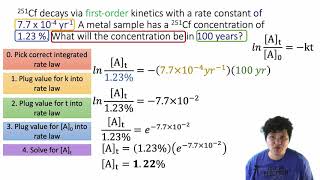
Problem 49b
Textbook Question
Textbook QuestionThe tabulated data show the concentration of AB versus time for this reaction: AB( g)¡A( g) + B( g) Time (s) [AB] (M) 0 0.950 50 0.459 100 0.302 150 0.225 200 0.180 250 0.149 300 0.128 350 0.112 400 0.0994 450 0.0894 500 0.0812 Determine the order of the reaction and the value of the rate constant. Predict the concentration of AB at 25 s.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
2864
views
4
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos