7. Gases
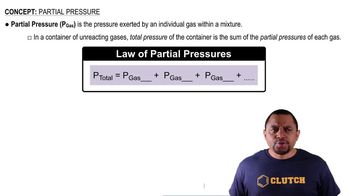
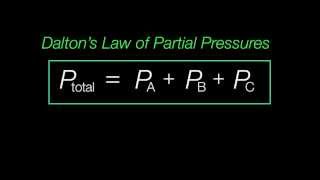
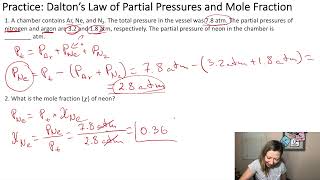
Partial Pressure
Problem 64
Textbook Question
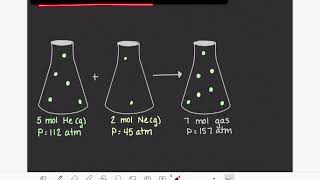
Textbook QuestionA 275-mL flask contains pure helium at a pressure of 752 torr. A second flask with a volume of 475 mL contains pure argon at a pressure of 722 torr. If we connect the two flasks through a stopcock and we open the stopcock, what is the partial pressure of argon?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
3109
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos