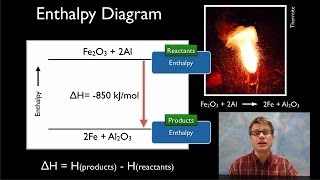
8. Thermochemistry
Enthalpy of Formation
Problem 143b
Textbook Question
Textbook QuestionMethanol 1CH3OH2 is made industrially in two steps from CO and H2. It is so cheap to make that it is being considered for use as a precursor to hydrocarbon fuels, such as methane 1CH42: Step 1. CO1g2 + 2 H21g2 S CH3OH1l2 ΔS° = - 332 J>K Step 2. CH3OH1l2 S CH41g2 + 1>2 O21g2 ΔS° = 162 J>K (a) Calculate ΔH° in kilojoules for step 1.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
452
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 6 videos