3. Chemical Reactions
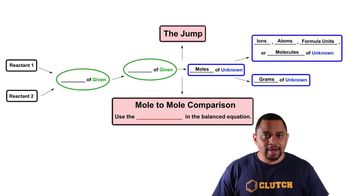
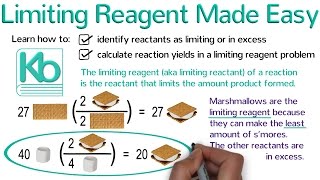
Limiting Reagent
Problem 105b
Textbook Question
Textbook QuestionWhen a mixture of 10.0 g of acetylene 1C2H22 and 10.0 g of oxygen 1O22 is ignited, the resulting combustion reaction produces CO2 and H2O. (c) How many grams of C2H2 are present after the reaction is complete?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
618
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos