7. Gases
Chemistry Gas Laws: Combined Gas Law

Get help from an AI Tutor
Ask a question to get started.
Problem 3
Textbook Question
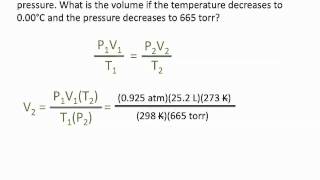
Textbook QuestionAssume that you have a gas cylinder with a movable piston filled with oxygen. The initial conditions are T = 250 K, n = 0.140 mol O2, and P = 1.00 atm. If the initial volume is 1.0 L, what is the volume when the temperature is increased to 400 K and the pressure is decreased to 0.75 atm? (LO 10.3) (a) 2.1 L (b) 1.2 L (c) 0.83 L (d) 1.6 L
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
415
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos