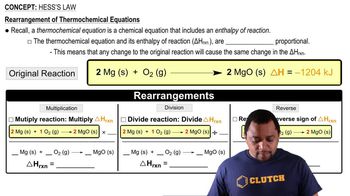
8. Thermochemistry
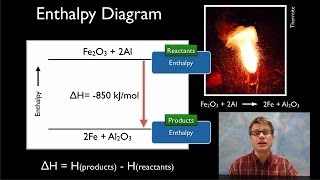
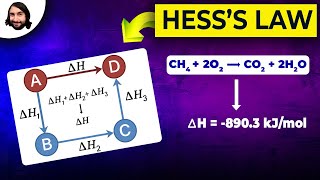
Hess's Law
Problem 28a
Textbook Question
Textbook QuestionThis reaction has an equilibrium constant of Kp = 2.2 * 106 at 298 K. 2 COF2(g) ⇌ CO2(g) + CF4(g) Calculate Kp for each reaction and predict whether reactants or products will be favored at equilibrium. c. 2 CO2(g) + 2 CF4(g) ⇌ 4 COF2(g)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
624
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos