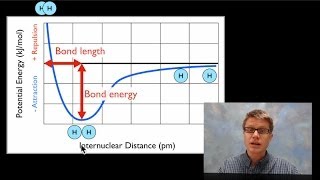
11. Bonding & Molecular Structure
Bond Energy
Problem 86
Textbook Question
Textbook Question(a) The nitrogen atoms in an N2 molecule are held together by a triple bond; use enthalpies of formation in Appendix C to estimate the enthalpy of this bond, D(N‚N). (b) Consider the reaction between hydrazine and hydrogen to produce ammonia, N2H41g2 + H21g2¡2 NH31g2. Use enthalpies of formation and bond enthalpies to estimate the enthalpy of the nitrogen– nitrogen bond in N2H4. (c) Based on your answers to parts (a) and (b), would you predict that the nitrogen–nitrogen bond in hydrazine is weaker than, similar to, or stronger than the bond in N2 ?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1576
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos