3. Chemical Reactions
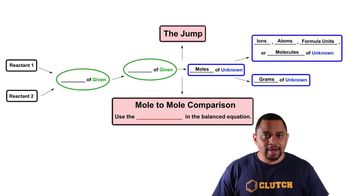
Limiting Reagent

Get help from an AI Tutor
Ask a question to get started.
Problem 74
Textbook Question
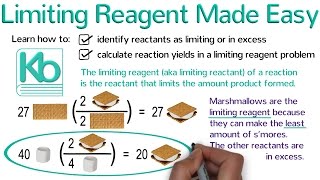
Textbook QuestionAssume that you have 1.39 mol of H2 and 3.44 mol of N2. How many grams of ammonia (NH3) can you make, and how many grams of which reactant will be left over? 3 H2 + N2 --> NH3
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
1121
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos