3. Chemical Reactions
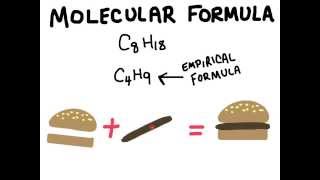
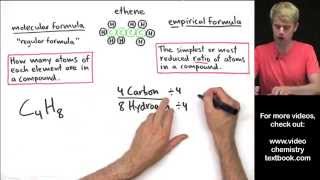
Molecular Formula
Problem 112
Textbook Question
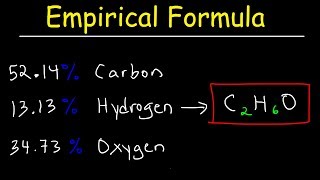
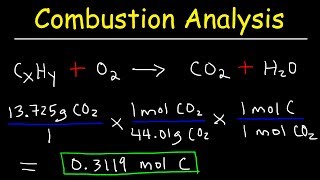
Textbook QuestionA pulverized rock sample believed to be pure calcium carbonate, CaCO3, is subjected to chemical analysis and found to contain 51.3% Ca, 7.7% C, and 41.0% O by mass. Why can't this rock sample be pure CaCO3?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
814
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos