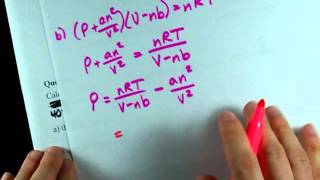7. Gases
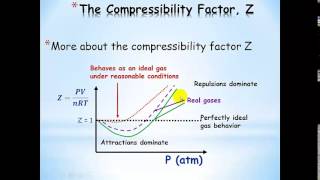
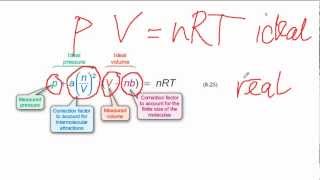
Van der Waals Equation

Get help from an AI Tutor
Ask a question to get started.
Problem 12b
Textbook Question
Textbook QuestionThe graph below shows the change in pressure as the temperature increases for a 1-mol sample of a gas confined to a 1-L container. The four plots correspond to an ideal gas and three real gases: CO2, N2, and Cl2. (b) Use the van der Waals constants in Table 10.3 to match the labels in the plot (A, B, and C) with the respective gases 1CO2, N2, and Cl22.

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
456
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos