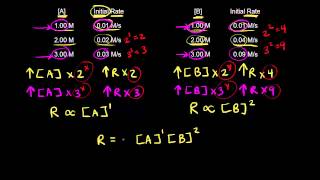15. Chemical Kinetics
Rate Law
Problem 40d
Textbook Question
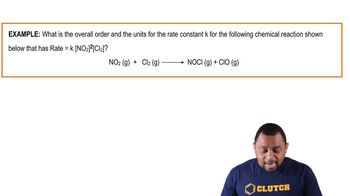
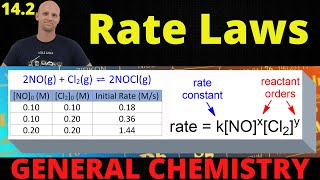
Textbook QuestionA reaction in which A, B, and C react to form products is zero order in A, one-half order in B, and second order in C. e. By what factor does the reaction rate change if [C] is doubled (and the other reactant concentrations are held constant)?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
2394
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos