16. Chemical Equilibrium
ICE Charts
Problem 74
Textbook Question
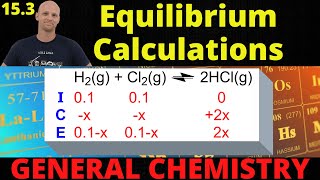
Textbook QuestionWhen 2.00 mol of SO2Cl2 is placed in a 2.00-L flask at 303 K, 56% of the SO2Cl2 decomposes to SO2 and Cl2 : SO2Cl21g2 Δ SO21g2 + Cl21g2 (a) Calculate Kc for this reaction at this temperature.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
1252
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos