3. Chemical Reactions
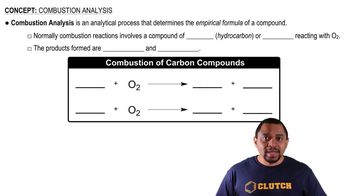
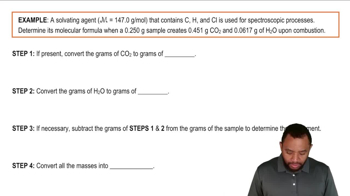
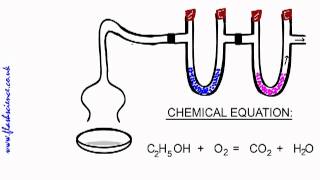
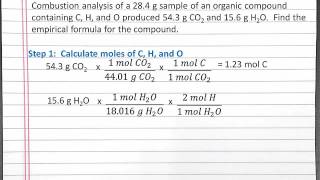
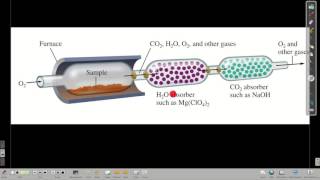
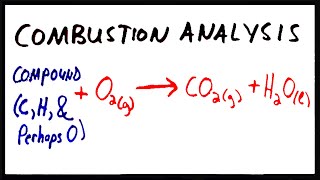
Combustion Analysis
Problem 56b
Textbook Question
Textbook Question(b) Nicotine, a component of tobacco, is composed of C, H, and N. A 5.250-mg sample of nicotine was combusted, producing 14.242 mg of CO2 and 4.083 mg of H2O. What is the empirical formula for nicotine?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
471
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos