11. Bonding & Molecular Structure
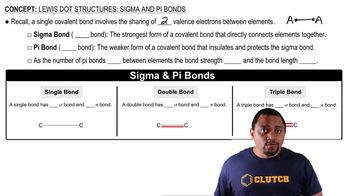
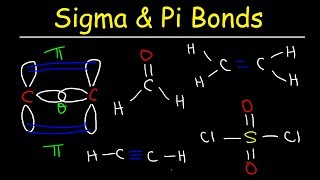
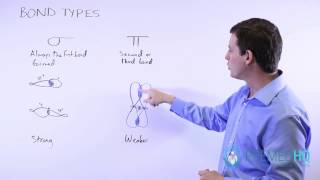
Lewis Dot Structures: Sigma & Pi Bonds
Problem 56
Textbook Question
Textbook Question(b) Imagine that you could hold two atoms that are bonded together, twist them, and not change the bond length. Would it be easier to twist (rotate) around a single s bond or around a double 1s plus p2 bond, or would they be the same?

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
423
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos