3. Chemical Reactions
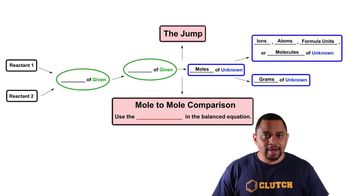
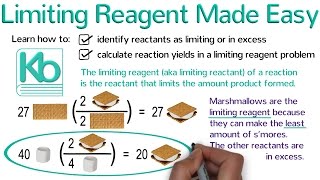
Limiting Reagent

Get help from an AI Tutor
Ask a question to get started.
Problem 44
Textbook Question
Textbook QuestionIron(II) sulfide reacts with hydrochloric acid according to the reaction: FeS(s) + 2 HCl(aq)¡FeCl2(s) + H2S(g) A reaction mixture initially contains 0.223 mol FeS and 0.652 mol HCl. Once the reaction has occurred as completely as possible, what amount (in moles) of the excess reactant remains?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
7592
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos