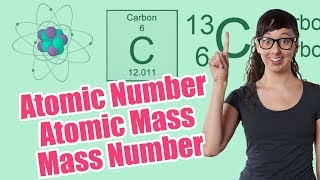2. Atoms & Elements
Atomic Mass
Problem 35
Textbook Question
Textbook QuestionIron has three major isotopes: 54Fe (atomic mass = 53.9396 u; abundance 5.85%), 56Fe (atomic mass = 55.9349 u; abundance 91.75%), and 57Fe (atomic mass = 56.9354 u; abundance 2.12 %). Calculate the atomic weight (average atomic mass) of iron.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
3719
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos