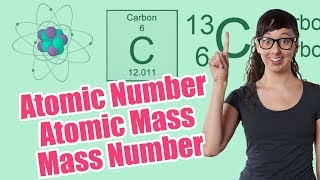2. Atoms & Elements
Atomic Mass
Problem 93
Textbook Question
Textbook QuestionThe nucleus of 6Li is a powerful absorber of neutrons. It exists in the naturally occurring metal to the extent of 7.5%. In the era of nuclear deterrence, large quantities of lithium were processed to remove 6Li for use in hydrogen bomb production. The lithium metal remaining after removal of 6Li was sold on the market. (b) The atomic masses of 6Li and 7Li are 6.015122 and 7.016004 u, respectively. A sample of lithium depleted in the lighter isotope was found on analysis to contain 1.442% 6Li. What is the average atomic weight of this sample of the metal?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
801
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos