Some theories of aging suggest that free radicals cause certain diseases and perhaps aging in general. As you know from the Lewis model, such molecules are not chemically stable and will quickly react with other molecules. According to certain theories, free radicals may attack molecules within the cell, such as DNA, changing them and causing cancer or other diseases. Free radicals may also attack molecules on the surfaces of cells, making them appear foreign to the body's immune system. The immune system then attacks the cells and destroys them, weakening the body. Draw Lewis structures for each free radical implicated in this theory of aging. c. OH

Free radicals are important in many environmentally significant reactions (see the Chemistry in the Environment box on free radicals in this chapter). For example, photochemical smog— smog that results from the action of sunlight on air pollutants— forms in part by these two steps:
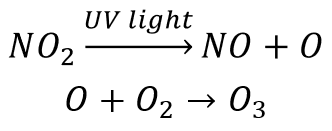
The product of this reaction, ozone, is a pollutant in the lower atmosphere. (Upper atmospheric ozone is a natural part of the atmosphere that protects life on Earth from ultraviolet light.) Ozone is an eye and lung irritant and also accelerates the weathering of rubber products. Rewrite the given reactions using the Lewis structure of each reactant and product. Identify the free radicals.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Free Radicals

Lewis Structures

Photochemical Reactions

Some theories of aging suggest that free radicals cause certain diseases and perhaps aging in general. As you know from the Lewis model, such molecules are not chemically stable and will quickly react with other molecules. According to certain theories, free radicals may attack molecules within the cell, such as DNA, changing them and causing cancer or other diseases. Free radicals may also attack molecules on the surfaces of cells, making them appear foreign to the body's immune system. The immune system then attacks the cells and destroys them, weakening the body. Draw Lewis structures for each free radical implicated in this theory of aging. d. CH3OO (unpaired electron on terminal oxygen)
If hydrogen were used as a fuel, it could be burned according to this reaction: H2(g) + 1/2 O2(g) → H2O(g) Use average bond energies to calculate ΔHrxn for this reaction.
If hydrogen were used as a fuel, it could be burned according to this reaction: H2(g) + 1/2 O2(g) → H2O(g) Use average bond energies to calculate ΔHrxn for the combustion of methane (CH4).
If hydrogen were used as a fuel, it could be burned according to this reaction: H2(g) + 1/2 O2(g) → H2O(g) Which fuel yields more energy per mole?
