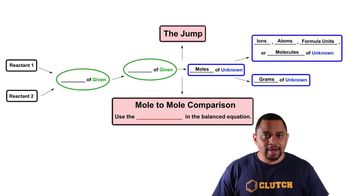
3. Chemical Reactions
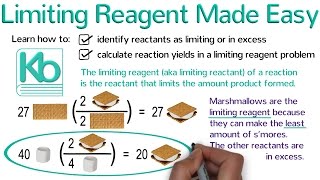
Limiting Reagent

Get help from an AI Tutor
Ask a question to get started.
Problem 47
Textbook Question
Textbook QuestionIron(III) oxide reacts with carbon monoxide according to the equation: Fe2O3(s) + 3 CO(g)¡2 Fe(s) + 3 CO2( g) A reaction mixture initially contains 22.55 g Fe2O3 and 14.78 g CO. Once the reaction has occurred as completely as possible, what mass (in g) of the excess reactant remains?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
14686
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos