7. Gases
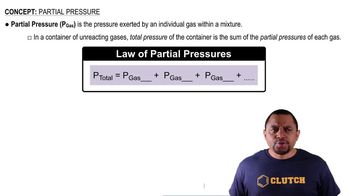
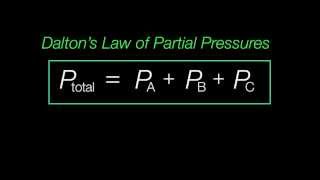
Partial Pressure
Problem 63
Textbook Question
Textbook QuestionA 1.20-g sample of dry ice is added to a 755 mL flask containing nitrogen gas at a temperature of 25.0 °C and a pressure of 725 mmHg. The dry ice sublimes (converts from solid to gas), and the mixture returns to 25.0 °C. What is the total pressure in the flask?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
2935
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos