8. Thermochemistry
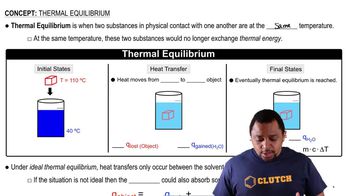
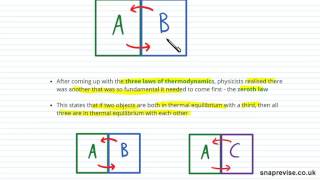
Thermal Equilibrium

Get help from an AI Tutor
Ask a question to get started.
Problem 45
Textbook Question
Textbook QuestionWe pack two identical coolers for a picnic, placing 24 12-ounce soft drinks and five pounds of ice in each. However, the drinks that we put into cooler A were refrigerated for several hours before they were packed in the cooler, while the drinks that we put into cooler B were at room temperature. When we open the two coolers three hours later, most of the ice in cooler A is still present, while nearly all of the ice in cooler B has melted. Explain this difference.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
921
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos