8. Thermochemistry
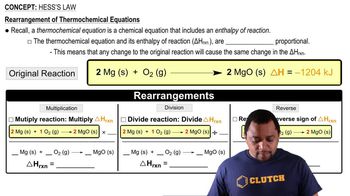
Hess's Law
Problem 79
Textbook Question
Textbook QuestionCalculate ΔHrxn for the reaction: Fe2O3(s) + 3 CO( g)¡2 Fe(s) + 3 CO2( g) Use the following reactions and given ΔH's: 2 Fe(s) + 32 O2( g)¡Fe2O3(s) ΔH = -824.2 kJ CO( g) + 12 O2( g)¡CO2( g) ΔH = -282.7 kJ
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
2691
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos