13. Liquids, Solids & Intermolecular Forces
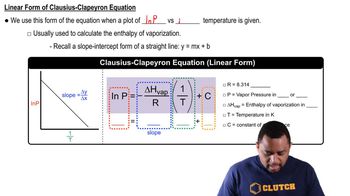
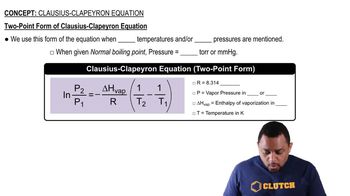
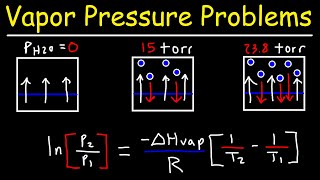
Clausius-Clapeyron Equation

Get help from an AI Tutor
Ask a question to get started.
Problem 85
Textbook Question
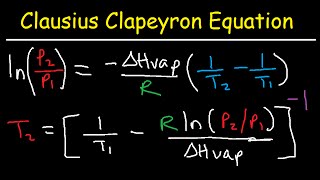
Textbook QuestionSupposethevaporpressureofasubstanceismeasuredattwo different temperatures. (a) By using the Clausius–Clapeyron equation (Equation 11.1) derive the following relationship between the vapor pressures, P1 and P2, and the absolute tem- peratures at which they were measured, T1 and T2: (b) Gasoline is a mixture of hydrocarbons, a component of which is octane (CH3CH2CH2CH2CH2CH2CH2CH3). Octane has a vapor pressure of 13.95 torr at 25 °C and a vapor pressure of 144.78 torr at 75 °C. Use these data and the equa- tion in part (a) to calculate the heat of vaporization of octane. (c) By using the equation in part (a) and the data given in part (b), calculate the normal boiling point of octane. Compare your answer to the one you obtained from Exercise 11.81. (d) Calculate the vapor pressure of octane at - 30 °C.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
477
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos