14. Solutions
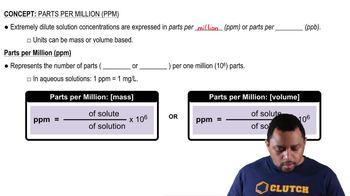
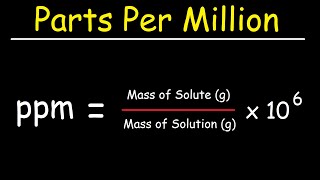
Parts per Million (ppm)

Get help from an AI Tutor
Ask a question to get started.
Problem 115b
Textbook Question
Textbook QuestionFederal regulations set an upper limit of 50 parts per million (ppm) of NH3 in the air in a work environment [that is, 50 molecules of NH31g2 for every million molecules in the air]. Air from a manufacturing operation was drawn through a solution containing 1.00 * 102 mL of 0.0105 M HCl. The NH3 reacts with HCl according to: NH31aq2 + HCl1aq2¡NH4Cl1aq2 After drawing air through the acid solution for 10.0 min at a rate of 10.0 L/min, the acid was titrated. The remaining acid needed 13.1 mL of 0.0588 M NaOH to reach the equivalence point. (a) How many grams of NH3 were drawn into the acid solution?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
310
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos