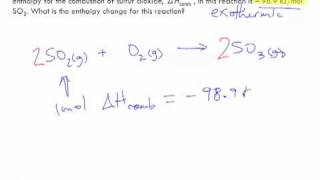8. Thermochemistry
Thermochemical Equations
Problem 48
Textbook Question
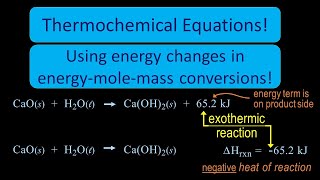
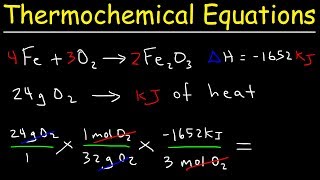
Textbook QuestionConsider the decomposition of liquid benzene, C6H61l2, to gaseous acetylene, C2H21g2: C6H61l2 ¡ 3 C2H21g2 H = +630 kJ (b) What is H for the formation of 1 mol of acetylene?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
235
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos