19. Chemical Thermodynamics
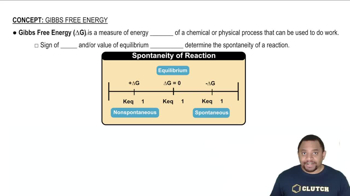
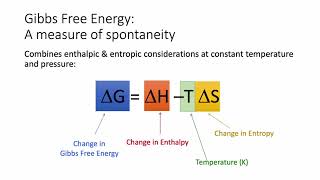
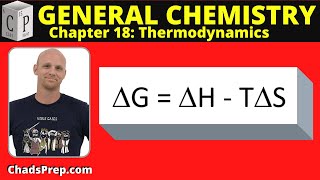
Gibbs Free Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 64
Textbook Question
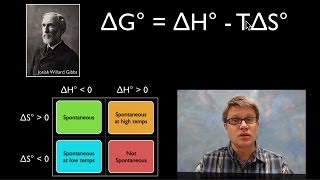
Textbook QuestionFrom the values given for ΔH° and ΔS°, calculate ΔG° for each of the following reactions at 298 K. If the reaction is not spontaneous under standard conditions at 298 K, at what temperature (if any) would the reaction become spontaneous? (a) 2 PbS1s2 + 3 O21g2¡2 PbO1s2 + 2 SO21g2 ΔH ° = -844 kJ; ΔS° = -165 J>K
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
2404
views
1
rank
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos