6. Chemical Quantities & Aqueous Reactions
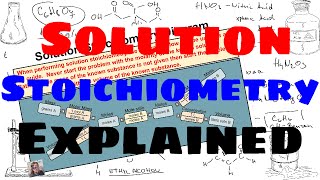
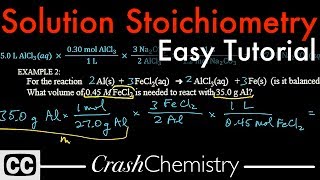
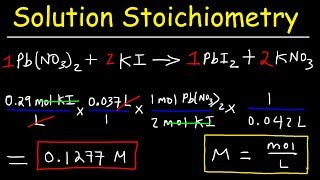
Solution Stoichiometry
Problem 141
Textbook Question
Textbook QuestionA 100.0 mL solution containing aqueous HCl and HBr was titrated with 0.1235 M NaOH. The volume of base required to neutralize the acid was 47.14 mL. Aqueous AgNO3 was then added to precipitate the Cl-and Br-ions as AgCl and AgBr. The mass of the silver halides obtained was 0.9974 g. What are the molarities of the HCl and HBr in the original solution?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
783
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos