Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. When samples of these are decomposed, the sulfur dioxide produces 3.49 g oxygen and 3.50 g sulfur, while the sulfur trioxide produces 6.75 g oxygen and 4.50 g sulfur. Calculate the mass of oxygen per gram of sulfur for each sample and show that these results are consistent with the law of multiple proportions.

Which statements are inconsistent with Dalton's atomic theory as it was originally stated? Why? a. All carbon atoms are identical. b. An oxygen atom combines with 1.5 hydrogen atoms to form a water molecule. c. Two oxygen atoms combine with a carbon atom to form a carbon dioxide molecule. d. The formation of a compound often involves the destruction of one or more atoms.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Dalton's Atomic Theory

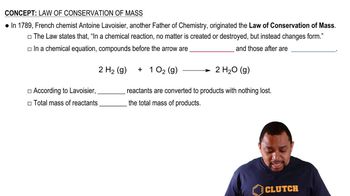
Law of Conservation of Mass
Stoichiometry and Atomic Ratios

Sulfur and fluorine form several different compounds including sulfur hexafluoride and sulfur tetrafluoride. Decomposition of a sample of sulfur hexafluoride produces 4.45 g of fluorine and 1.25 g of sulfur, while decomposition of a sample of sulfur tetrafluoride produces 4.43 g of fluorine and 1.87 g of sulfur. Calculate the mass of fluorine per gram of sulfur for each sample and show that these results are consistent with the law of multiple proportions.
Which statements are consistent with Dalton's atomic theory as it was originally stated? Why? a. Sulfur and oxygen atoms have the same mass. b. All cobalt atoms are identical. c. Potassium and chlorine atoms combine in a 1:1 ratio to form potassium chloride. d. Lead atoms can be converted into gold.
Which statements are consistent with Rutherford's nuclear theory as it was originally stated? Why? a. The volume of an atom is mostly empty space. b. The nucleus of an atom is small compared to the size of the atom. c. Neutral lithium atoms contain more neutrons than protons. d. Neutral lithium atoms contain more protons than electrons.
Which statements are inconsistent with Rutherford's nuclear theory as it was originally stated? Why? a. Since electrons are smaller than protons, and since a hydrogen atom contains only one proton and one electron, it must follow that the volume of a hydrogen atom is mostly due to the proton. b. A nitrogen atom has 7 protons in its nucleus and 7 electrons outside of its nucleus. c. A phosphorus atom has 15 protons in its nucleus and 150 electrons outside of its nucleus. d. The majority of the mass of a fluorine atom is due to its 9 electrons.
A chemist in an imaginary universe, where electrons have a different charge than they do in our universe, performs the Millikan oil drop experiment to measure the electron's charge. The charges of several drops are recorded here. What is the charge of the electron in this imaginary universe?
Drop # Charge
A –6.9×10–19 C
B –9.2×10–19 C
C –11.5×10–19 C
D –4.6×10–19 C
