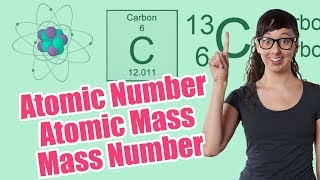2. Atoms & Elements
Atomic Mass
Problem 95
Textbook Question
Textbook QuestionThe element chromium (Cr) consists of four naturally occurring isotopes with atomic masses 49.9460, 51.9405, 52.9407, and 53.9389 u. The relative abundances of these four isotopes are 4.3, 83.8, 9.5, and 2.4%, respectively. From these data, calculate the atomic weight of chromium.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1798
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos